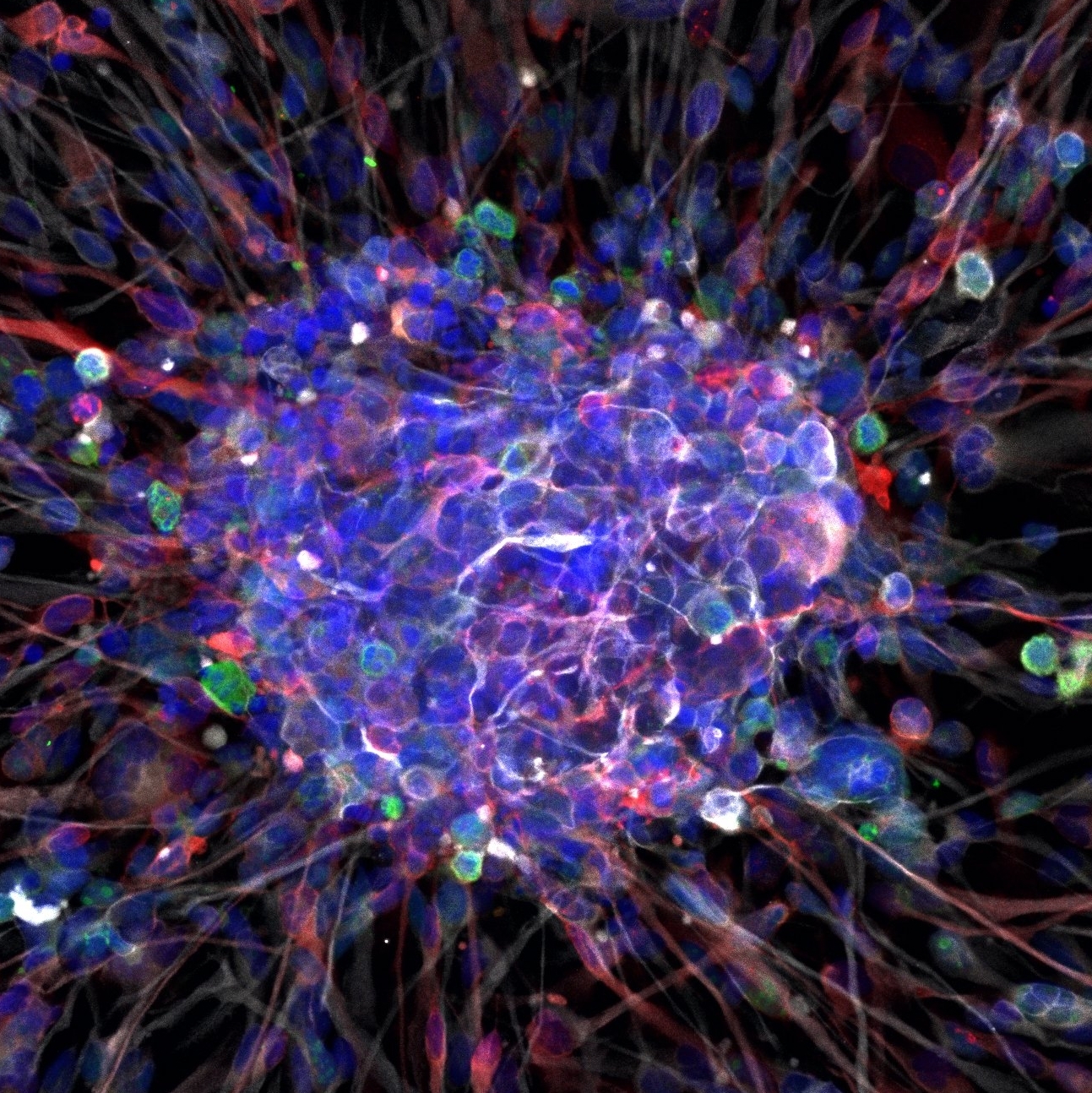
Our laboratory studies the regulation of stem cell function in malignant brain tumors called gliomas. By definition, stem cells possess two fundamental properties: self-renewal, the ability to preserve stemness; and multi-potency, the ability to differentiate to different types of progeny. Our group investigates the molecular mechanisms that govern self-renewal and differentiation potential of glioma stem cells, as well as their putative cell-of-origin, the neural stem cells of the brain.
A major project in our laboratory aims to identify distinct classes of cancer stem cells in glioblastoma. Glioblastoma is an aggressive form of brain cancer, in which stem-like cells termed glioblastoma stem cells (GSCs) can recapitulate the entire tumor, while remaining resistant to chemotherapy and radiation. To study GSC biology, we obtain primary human GBM tissue from operative specimens, culture it and inject it into the mouse brain to generate tumor xenografts. Using genetic techniques and lentiviral vectors, we interrogate distinct types of human GSCs for the lineages that they generate in vitro and in vivo, molecular markers, metabolic profile and response to treatment. As a result of this effort, we recently identified an adhesion G protein-coupled receptor (GPCR), GPR133, which is required for glioblastoma growth. We are currently elucidating basic mechanisms of action of GPR133, but also developing it translationally as a novel therapeutic target. In addition, we have extended our studies to other members of the adhesion GPCR family, such as are CD97, which are also involved in glioblastoma pathogenesis.
An additional area of research in the laboratory focuses on understanding the early steps of gliomagenesis. Over the past few years, brain tumor sequencing has indicated that neomorphic mutations in the metabolic enzyme isocitrate dehydrogenase 1 (IDH1) are highly prevalent in low-grade glioma, a slow-growing precursor to glioblastoma. We recently used a human neural stem cell platform to show that tumor initiation by mutant IDH1 is mediated by 3D chromatin conformation changes, which result in reorganization of transcription factor networks that regulate differentiation. We are currently pursuing genetic, biochemical and pharmacologic approaches to elucidate epigenetic changes during glioma initiation and identify related dependencies of tumor cells. In addition, we have extended this work to human neurogenesis and have identified dynamic regulation of 3D chromatin organization and enhancer activity during neural development.
A new and exciting area of research in our group focuses on understand the role of tumor cell-intrinsic calcium waves on glioma growth. We can image these waves both in vitro, in patient-derived glioblastoma cultures, and in tumor xenografts in vivo. Current efforts include understanding the mechanisms that generate these waves, as well the downstream signaling cascades.
The role of adhesion GPCRs in glioma biology
We recently described that GPR133, an orphan member of the adhesion family of G protein-coupled receptors (GPCR), is necessary for glioblastoma growth. GPR133 is expressed in all glioblastomas, regardless of molecular subtype, but is absent from normal brain tissue. This expression pattern suggests a favorable therapeutic profile. We are currently elucidating basic mechanisms of action of GPR133, and other adhesion GPCRs, such as CD97, but also developing them translationally as novel therapeutic targets.
Developmentally regulated 3D chromatin architecture and enhancer activation in human neurogenesis
We discovered that transcription of SOX2, which encodes a transcription factor critical to neural progenitor self-renewal and multipotency, is regulated by a distal enhancer through 3D chromatin folding. Both the enhancer activation and the chromatin looping occur specifically in neural progenitors but not embryonic stem cells or other germ layers. We are using CRISPR and CRISPRi/a to perturb this system across development, in order to identify mechanisms critical to human neurogenesis.
Dynamic regulation of 3D chromatin organization and enhancer activation in glioma initiation
We are employing genetic, biochemical and pharmacologic approaches to dissect the epigenetic mechanisms related to 3D chromatin organization that are responsible for glioma initiation, using human embryonic stem cell models and patient-derived tumor cells. The work involves 3D chromatin and enhancer characterization and CRISPR perturbation.
Calcium dynamics and signaling in glioma
Glioma cells manifest robust, tumor cell-intrinsic calcium waves that we can image in vitro and in vivo. We believe that these waves are relevant to tumor growth. A new and and exciting project in the lab focuses on understanding the mechanisms underlying these waves, their importance to tumor biology, and the signaling mechanisms initiated by calcium flux in glioma.
Take a look at our calcium imaging of patient-derived GBM cells with GCaMP6s:
https://twitter.com/placantonakilab/status/1426221003559120896?s=21
Novel vulnerabilities in GBM
We are using CRISPR and CRISPRi screening to identify novel vulnerabilities in glioblastoma (GBM). In particular, we are working on identifying a) genes whose knockout is synthetically lethal with existing GBM therapies; and b) enhancer elements critical for tumor growth.
Heterogeneity in the cancer stem cell population in GBM
Glioblastoma (GBM) is the most aggressive form of glioma. Over the past decade, several lines of evidence have indicated that not every cell in these tumors is equal. Another way to state this is that some tumor cells in GBM are more important than the rest. In biological terms, this inequality translates into a cellular hierarchy, whose apex is occupied by cancer stem cells.
Tissue homeostasis is maintained by stem cells that generate defined lineages of specialized differentiated cells. Not surprisingly, GBM tumors are remarkably complex at the histologic level, which begs the question: Does a single cell type with stem-like properties produce the entire spectrum of tumor lineages and histologies or is the cancer stem cell population heterogeneous?
Using human GBM cultures, we recently discovered that within any given GBM tumor there are multiple tumor cell types that fulfill stem cell criteria. These cell types manifest striking transcriptional and metabolic differences, in addition to discreet differentiation programs that allow them to adapt to diverse microenvironmental conditions and shape the niches where they reside.